


For 63Cu, the atomic mass is less than 63, so this must be the dominant factor. Sulfur is a chemical element with atomic number 16 which means there are 16 protons in its nucleus. A nucleus with greater binding energy has lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc 2. The nuclear binding energy varies between nuclei.This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
The neutron is slightly heavier than the proton.There are two reasons for the difference between mass number and isotopic mass, known as the mass defect: For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in its nuclear ground state is 62.91367 u. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.įor 12C, the atomic mass is exactly 12u, since the atomic mass unit is defined from it. suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an OO double bond, as shown in the. An excited state is a state in which an electron jumps to. Sulfurs electron configuration is 2-8-6 according to the Reference Table. pdf), Text File ( Stable Electron Configurations All atoms react to achieve noble gas configuration What is the electron configuration of a sulfur atom 1s22s22p63s23p4 6 valence electrons For calcium, which has an atomic number of 20 and therefore 20 electrons, find calcium on For calcium, which has an atomic number of 20 and therefore 20.
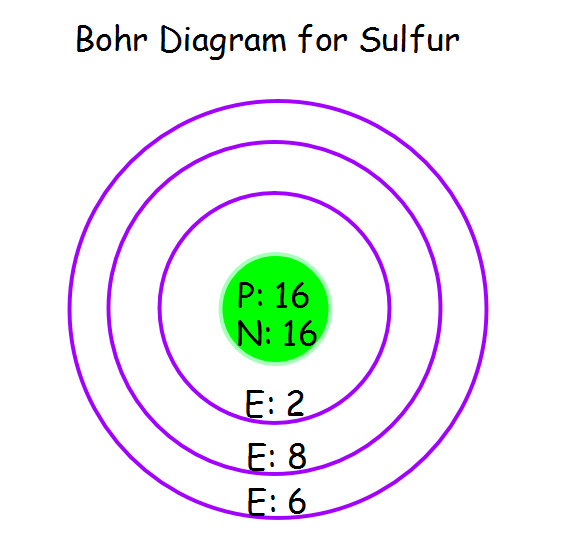
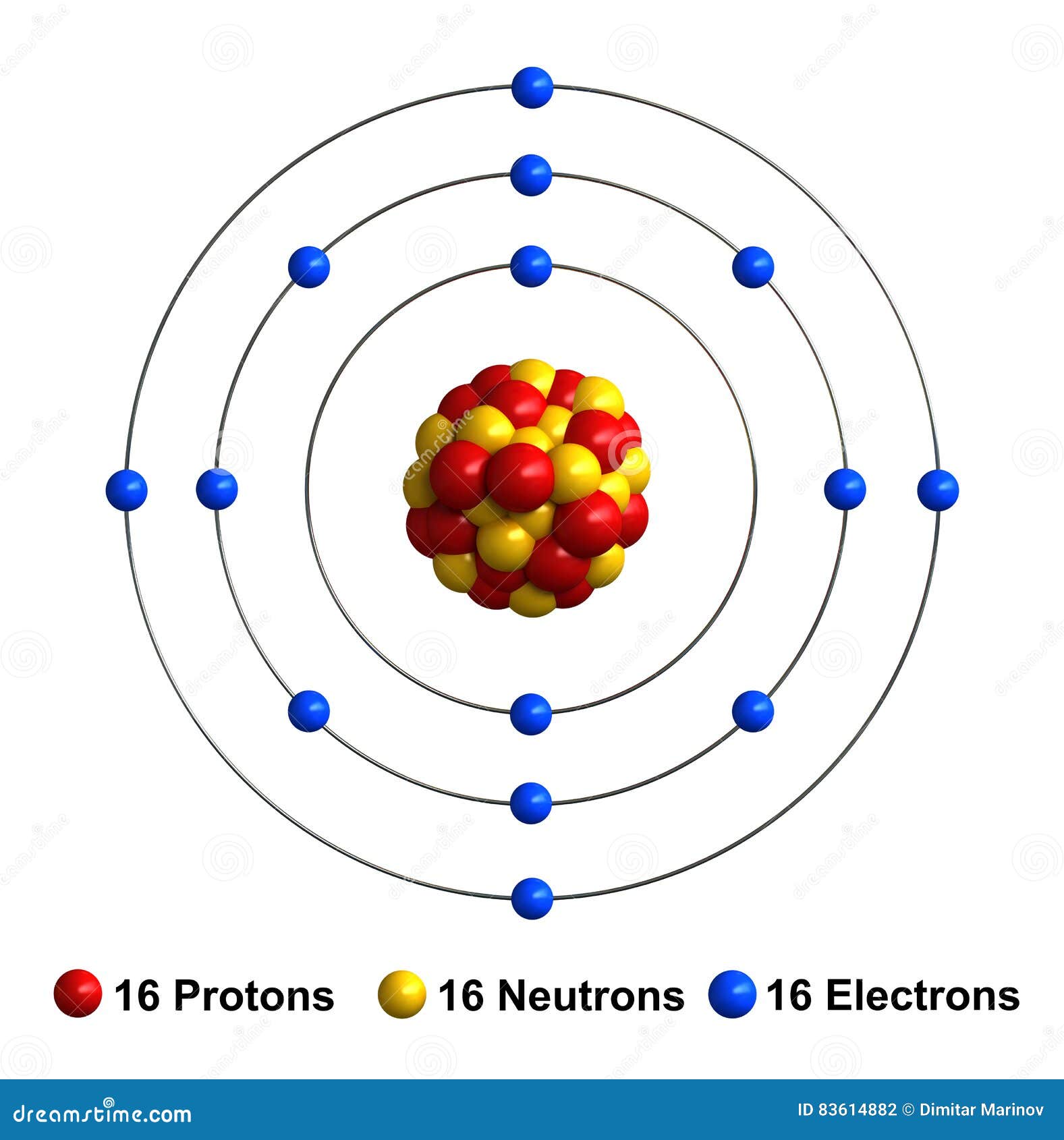
One atomic mass unit is equal to 1.66 x 10 -24 grams. Which electron configuration represents the electrons in an atom of sulfur in an excited state (1) 2 8 6 (2) 2 7 7 (3) 2 8 7 (4) 2 7 8 Explanation: We must first look at the Periodic Table on the Reference Table, and find Sulfur. The unit of measure for mass is the atomic mass unit (amu). Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance. Note that each element may contain more isotopes. How does the atomic number determine the chemical behavior of atoms? Atomic Mass of Sulfur Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure.


 0 kommentar(er)
0 kommentar(er)
